
Atomic Structure Worksheets⁚ A Comprehensive Guide
Atomic structure worksheets are valuable tools for students to solidify their understanding of atoms and their fundamental components. These worksheets often present questions and problems that require students to apply their knowledge of atomic structure to answer questions about atomic properties, such as atomic number, mass number, and electron configuration.
These worksheets are designed to help students develop a strong foundation in atomic theory, which is essential for success in chemistry and other science fields.
Introduction
Delving into the realm of chemistry, understanding the building blocks of matter, atoms, is paramount. Atomic structure worksheets serve as valuable tools for both teachers and students, providing a structured platform to explore the intricacies of the atom. These worksheets encompass a wide range of exercises, from basic definitions and diagrams to more complex calculations and problem-solving scenarios. They cater to diverse learning styles, offering a visual, interactive, and engaging approach to mastering this fundamental concept.
The availability of atomic structure worksheets with answers is a boon for students, allowing them to self-assess their understanding, identify areas requiring further attention, and solidify their grasp on the subject. These worksheets are particularly beneficial for independent learning, homework assignments, and test preparation. They provide a comprehensive guide to the key components of an atom, including protons, neutrons, and electrons, and explore concepts such as atomic number, mass number, and electron configuration.
By engaging with these worksheets, students gain a deeper appreciation for the fundamental principles governing atomic structure, laying the groundwork for a more comprehensive understanding of chemistry and related fields.
Understanding Atomic Structure
The atom, the fundamental building block of matter, is a fascinating microcosm of complexity. Its structure, while seemingly simple, underpins the very nature of the elements and their interactions. To grasp the essence of chemistry, a solid understanding of atomic structure is essential.
Atomic structure worksheets are invaluable tools for unraveling the mysteries of the atom. They offer a structured and interactive approach to exploring the key components of an atom ⸺ protons, neutrons, and electrons. These worksheets guide students through the concepts of atomic number, which represents the number of protons in an atom’s nucleus, and mass number, which reflects the total number of protons and neutrons.
By engaging with these worksheets, students delve into the intricacies of how these subatomic particles interact and influence the properties of an element. They learn about the nucleus, the atom’s dense core, and the surrounding electron cloud, where electrons orbit the nucleus in specific energy levels.
Understanding atomic structure lays the foundation for comprehending the periodic table, chemical bonding, and the reactions that govern the world around us.
Key Components of an Atom
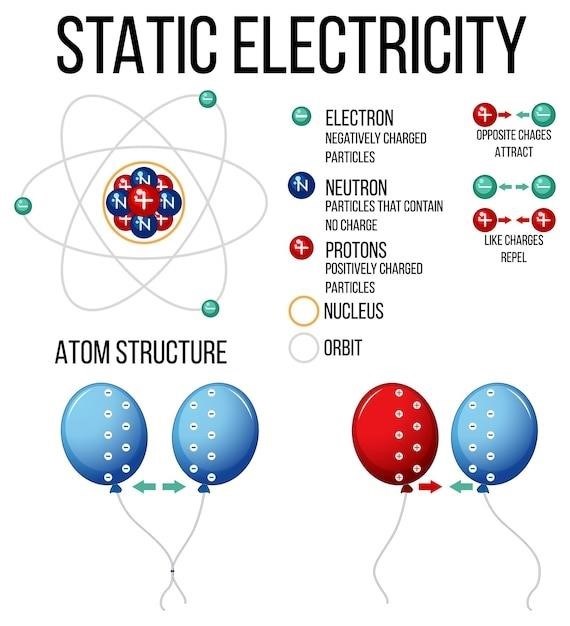
Atoms, the fundamental building blocks of all matter, are composed of three primary subatomic particles⁚ protons, neutrons, and electrons. These particles, while incredibly small, play crucial roles in determining an atom’s properties and how it interacts with other atoms.
Protons, positively charged particles, reside within the atom’s nucleus, the dense core at the center. The number of protons in an atom defines its atomic number, which uniquely identifies an element on the periodic table.
Neutrons, neutral particles, also reside in the nucleus, contributing to the atom’s mass. While they don’t carry a charge, neutrons play a significant role in nuclear stability.
Electrons, negatively charged particles, orbit the nucleus in a cloud-like region known as the electron cloud. These electrons occupy specific energy levels, or shells, and their arrangement determines the atom’s chemical behavior and its ability to form bonds with other atoms.
Atomic structure worksheets often feature diagrams and exercises that help students visualize these components and their arrangement within the atom.
Atomic Number and Mass Number
Atomic number and mass number are two fundamental concepts that help define and characterize atoms. These numbers provide valuable information about the composition and identity of an atom.
The atomic number, denoted by the letter ‘Z’, represents the number of protons in an atom’s nucleus. It’s a defining characteristic of an element, meaning all atoms of a particular element have the same atomic number. For instance, all carbon atoms have an atomic number of 6, indicating they possess 6 protons.
The mass number, denoted by the letter ‘A’, represents the total number of protons and neutrons in an atom’s nucleus. Since protons and neutrons contribute almost all of an atom’s mass, the mass number provides a close approximation of an atom’s mass.
Atomic structure worksheets often present exercises that require students to determine atomic number and mass number based on the number of protons, neutrons, and electrons in an atom.
Understanding atomic number and mass number is crucial for comprehending the organization of the periodic table, the relationships between elements, and the behavior of atoms in chemical reactions.
Isotopes and Their Significance
Isotopes are atoms of the same element that share the same atomic number (number of protons) but differ in their mass number (number of neutrons). This means isotopes of a given element have the same number of protons but a varying number of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon. Carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons.
Isotopes play a crucial role in various fields, including chemistry, physics, and biology. They are utilized in radioactive dating to determine the age of ancient artifacts and fossils. Some isotopes, known as radioisotopes, are unstable and undergo radioactive decay, emitting particles and energy. This property makes radioisotopes useful in medical imaging and treatment, industrial applications, and scientific research.
Atomic structure worksheets often include questions that require students to identify isotopes based on their atomic and mass numbers, understand the concept of radioactive decay, and explore the applications of isotopes in different fields. By working through these exercises, students gain a deeper understanding of the diversity within elements and the significance of isotopes in various scientific disciplines.
Electron Configuration and Orbitals
Electron configuration describes the arrangement of electrons within an atom’s energy levels and orbitals. It follows specific rules, such as the Aufbau principle, which dictates that electrons fill orbitals in order of increasing energy. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins.
Orbitals are regions of space around the nucleus where there is a high probability of finding an electron. They are characterized by specific shapes and energy levels; The principal quantum number (n) defines the energy level, while the azimuthal quantum number (l) describes the shape of the orbital.
Electron configuration worksheets challenge students to determine the electron configuration of various elements using the periodic table as a guide. They may also be asked to draw orbital diagrams, which visually represent the arrangement of electrons within orbitals. Understanding electron configuration is crucial for predicting chemical reactivity, bonding behavior, and the properties of elements.
Bohr Model and Its Limitations
The Bohr model, proposed by Niels Bohr in 1913, provided a simplified yet groundbreaking description of atomic structure. It envisioned the atom as a miniature solar system, with electrons orbiting the nucleus in specific circular paths called energy levels.
The model successfully explained the line spectra of hydrogen, where electrons jump between energy levels, emitting or absorbing light of specific wavelengths.
Despite its success, the Bohr model had limitations. It failed to account for the spectra of atoms with more than one electron and couldn’t explain the finer details of atomic spectra, such as the splitting of spectral lines in magnetic fields. Furthermore, it couldn’t explain the chemical bonding between atoms, which involves the sharing or transfer of electrons.
While the Bohr model is a useful starting point for understanding atomic structure, it is important to recognize its limitations and acknowledge the more sophisticated quantum mechanical model that emerged later.
Quantum Mechanical Model
The quantum mechanical model, developed in the early 20th century, revolutionized our understanding of atomic structure. Unlike the Bohr model, which treated electrons as particles orbiting the nucleus in defined paths, the quantum mechanical model describes electrons as wave-like entities.
This model employs the concept of atomic orbitals, which are three-dimensional regions around the nucleus where electrons are most likely to be found. Each orbital has a specific energy level and shape, and can hold a maximum of two electrons with opposite spins.
The quantum mechanical model is based on the Schrödinger equation, a complex mathematical equation that describes the behavior of electrons in atoms. Solving this equation provides a set of four quantum numbers that describe the energy, shape, and spatial orientation of an electron in an atom. These numbers are crucial for understanding electron configuration and predicting atomic properties.
The quantum mechanical model accurately explains the spectra of atoms with multiple electrons and provides a framework for understanding chemical bonding and molecular structure. It is a cornerstone of modern chemistry and physics, providing a sophisticated and comprehensive picture of the atom.
Applications of Atomic Structure
The understanding of atomic structure has profound implications across various scientific disciplines and technological advancements. It serves as the foundation for comprehending the behavior of matter at the molecular and macroscopic levels, leading to numerous applications in fields such as chemistry, materials science, and nuclear physics.
In chemistry, atomic structure is instrumental in explaining chemical bonding, predicting reactivity, and understanding the properties of compounds. The knowledge of electron configuration and orbital interactions allows chemists to design and synthesize new materials with specific properties.
Materials science leverages atomic structure to develop novel materials with enhanced properties, such as strength, conductivity, and optical characteristics. By manipulating the arrangement and interactions of atoms, scientists can create materials with tailored functionalities for applications ranging from electronics and aerospace to medicine and energy production.
Nuclear physics relies heavily on atomic structure to understand nuclear reactions, radioactive decay, and the properties of isotopes. This knowledge is essential for developing nuclear energy, medical imaging techniques, and nuclear weapons.
In conclusion, atomic structure is a fundamental concept that has revolutionized our understanding of matter and its properties. Its applications extend far beyond the realm of theoretical science, playing a vital role in shaping modern technologies and advancing scientific knowledge.
Resources for Further Learning
For those seeking to delve deeper into the fascinating world of atomic structure, numerous resources are available to enhance understanding and exploration.
Online platforms, such as Khan Academy and Coursera, offer comprehensive courses and tutorials on atomic structure, covering topics from basic concepts to advanced quantum mechanics. These platforms provide interactive learning experiences, including videos, quizzes, and practice problems, making them ideal for self-paced learning.
Textbooks, such as “Chemistry⁚ The Central Science” by Theodore L. Brown, H. Eugine LeMay Jr., and Bruce E. Bursten, provide in-depth explanations and examples of atomic structure and its applications. These books are valuable resources for students seeking a comprehensive understanding of the subject.
Scientific journals, such as “Journal of Physical Chemistry” and “Nature Chemistry,” publish cutting-edge research on atomic structure and its implications for various fields. These journals offer insights into the latest discoveries and advancements in the field.
Finally, engaging in discussions and collaborations with other learners and experts can foster a deeper understanding of atomic structure. Online forums, scientific societies, and research groups provide opportunities for interactive learning and knowledge sharing.
In conclusion, atomic structure worksheets serve as valuable tools for students to master the fundamental concepts of atomic theory. By engaging with these worksheets, students develop a solid understanding of the basic components of an atom, including protons, neutrons, and electrons. They learn to apply their knowledge to determine atomic number, mass number, and electron configurations.
These worksheets also introduce students to the significance of isotopes and their role in various fields, including nuclear chemistry and medicine. By tackling questions and problems related to atomic structure, students enhance their critical thinking skills and deepen their understanding of the fundamental building blocks of matter.
Whether used in classroom settings or for independent study, atomic structure worksheets play a crucial role in fostering a strong foundation in chemistry and related scientific disciplines. They provide a hands-on approach to learning, enabling students to visualize and interact with atomic concepts, ultimately leading to a more comprehensive and engaging understanding of the world around us.